Explore and deepen our understanding on the tissue regeneration technology.
BusinessToday’s interview with Dr. GK Ananda and Dr Lim Jing on the aforementioned technology.
BusinessToday: Can you provide us with a brief history of tissue regeneration technology and how it grew to become a multi-billion industry worldwide/local?
Dr. Ananda: Tissue regeneration technology has been a widely discussed topic for the past 3 decades. Its framework provides us with concepts that enable the regrowth of parts of the body or bones without having to graft bone from another part of our body. Due to the potential of this technology and the clinical evidence supporting its adoption in clinical practice, interest in this technology has surged considerably. Today, it is a billion-dollar industry and is projected to grow to $109.9 billion by 2023 with a compound annual growth rate of 34.8%.
BusinessToday: What are some of the benefits of tissue regeneration technology compared to more conventional/traditional treatment methods?
Dr. Ananda: Conventional treatment methods may involve the use of the patients’ own bone or issue that is taken from another part of the body, also known as autologous bone grafting. This technique, while considered a ‘gold standard’, can be substantially morbid to the patient due to a second surgical site. In fact, there are published clinical evidence indicating donor site morbidity.
Tissue regeneration technology is a viable alternative for surgeons as it avoids creating a second surgical site and hence helps reduce patient morbidity.
Additionally, regenerative implants that use bioresorbable materials are able to avoid complications that are commonly associated with permanent implants, such as late infections and delayed exposure of the implant through the skin.
BusinessToday: What are some of the challenges of tissue regeneration technology?
Dr. Ananda: While tissue regeneration technology is certainly revolutionary and can be used to save the lives of many, one of the main challenges is in making it available for clinical application.
On this note, there are 2 sub-branches of tissue engineering that we would like to highlight: in-vitro and in-situ engineering.
In-vitro tissue engineering is the process of growing tissues or organs outside of the body, then putting them back inside to ultimately improve health and change lives is slowly but surely becoming a reality. However, finding cells that are acceptable to our immune system is not a trivial task. Furthermore, there are also challenges in terms of the availability and scaling-up capabilities of in-vitro tissue engineering. Cost-effectiveness, preservation and handling also present problems. It takes time to grow cells and tissue, and there is no guarantee the cells and tissues grown are viable and not mutated. Lastly, manipulating cells in an external environment is a highly regulated activity and may hamper its availability for clinical application.
On the other hand, in-situ engineering, the method Osteopore uses, allows for quicker application and enables Osteopore to make a more immediate impact on patient’s recovery, globally. It is comparatively more cost-effective and reproducible as compared to in-vitro tissue engineering and we can consistently repeat the same desired results. With in-situ tissue engineering, we look at medical products that interface with bone tissue, blood vessels and cells – working in harmony with how our body already functions. It essentially harnesses the native regenerative potential of the body to regenerate tissue, as opposed to creating it wholesale in an in-vitro environment.
Rather than implanting cells into the body from an external source, in-situ tissue engineering looks to recruit endogenous stem cells (cells created within a living organism) to the site of an injury by using biomaterial with 3D microarchitecture and/or growth-factor-based cues to enhance healing. That said, when it comes to in-situ tissue engineering, any construct that is implanted into the body is not an immediately fully-functional, full-size replacement of the lost tissue – rather, the constructs look to grow with and enhance the pre-existing regenerative capabilities of the human body.
Testing has confirmed that our microarchitecture balances strength and cell proliferation with consistent effectiveness. That said, the challenge presents itself in determining the most effective trade-off between scaffold strength and porosity. Based on the clinical outcomes of our device, we have demonstrated that the technology is able to marry both these properties effectively.
BusinessToday: How does Osteopore’s tissue regeneration technology work?
Dr. Ananda: At Osteopore, we combine 3D printing with bioresorbable polycaprolactone (PCL) to create a conducive environment for bone cells and blood vessels to grow into. As PCL degrades and is absorbed by the body over a period of 18-24 months, natural tissue regeneration occurs and eventually the patient’s own natural bone takes over.
After performing its function, our implants then will metabolize into water and carbon dioxide, leaving only natural, healthy bone. This makes our implants particularly well-suited for craniofacial, orthopaedic, and dental surgeries. It disappears over time, thereby avoiding almost all post-surgery complications commonly associated with permanent implants.
BusinessToday: Can you shed some light on how this tech can be used in various scenarios and circumstances?
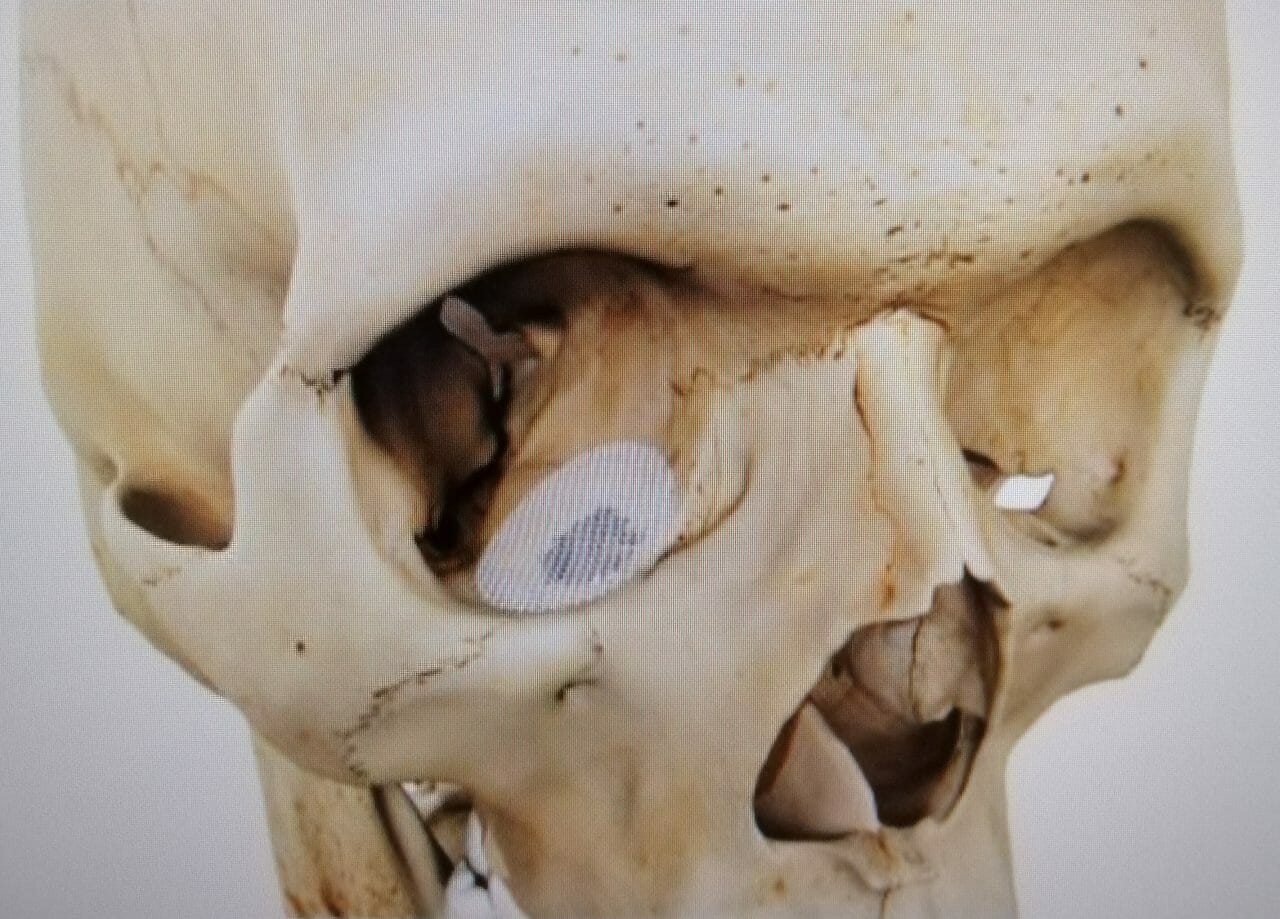
Dr Lim Jing: Some patients may experience bone loss due to accidents or diseases. In these situations, the amount of bone removed may be too much for the bone to repair itself. This is where our technology becomes the enabler for bone regeneration, leveraging on the 3-dimensional architecture to facilitate tissue and blood vessel ingrowth, thereby assisting bone to heal itself. Some applications include the regeneration of bone in the skull that arise as a result of burr hole surgery or craniotomy, and of the easily broken thin bone, called the orbital floor, that supports the eyeball. We have proven this technology through long-term clinical follow-ups that are published in renowned international journals. We have also published clinical experiences on life-changing surgeries in patients who may have to face amputation without our technology.
We are aware that our technology is equally capable of functioning in other areas such as augmenting or correcting the nose structure and its looks – this is a clinical application that was co-developed with Korean surgeons working in this area.
BusinessToday: Can you provide some insights and opinions on the future of this tech?
Dr GK Ananda uses Osteopore Regenerative Mesh in Orbital Floor Reconstruction (see photo below). We have studies to show that our mesh in this application minimizes complications, such as orbit compartment syndrome (rise in orbital pressure) of permanent implants as evidenced by a 10-year clinical series.
Dr Ananda: At the moment, in my practice, I use the Ostoemesh. Perhaps regenerative implant in the oral maxillofacial application could involve implants with a more anatomical shape (more 3Ds hape/structure) that can be inserted in the defect instead of a somewhat flat implant.
BusinessToday: Please share with us some of your experience working with tissue regenerative technology
Dr Ananda: I have been using Osteomesh for around 5 years. There is always a risk of infection when there is a permanent object in the body. Osteomesh resorbs after a period of time, and so far, my experience with in 50 cases with the mesh is that there have been no issues with infection. There were, however, a few cases where there was some displacement of the mesh.
Also, on its ease of use – I can trim or cut the mesh easily to fit the defect. It helps me cut my surgery time.
In cases where I use Osteomesh, I still follow my standard follow-up protocols I have for other implants, so I cannot really attest to the reduction in the number of follow-ups with regenerative products. But I don’t really receive complaints about electrical sensations from my patients, which happens with metal implants (and has been proven in studies).